
scancare instrument tracking
Designed to comply with AS 5369 & NSQHS Standard 3 - for Infection Control, ScanCARE tracks and manages:
- Sterilization Process Tracking
- Wash and disinfection tracking
- Sterilizer and Washer Challenge Tests
- Annual Performance Qualifications
- Surgical device testing
- Prosthesis & Consumables
- Staff Activity
- Non conformance management and reporting
- Compatible and Preferred Sterilizer & Washer Cycle Types
- Fast Track Instruments
- Instrument Service & History
- Attach IFU's. Safety Data Sheets, Instrument and Tray images and videos

compliance guarantee
Designed and developed in Australia to comply with AS/NZS Standards, ScanCARE Activity Tracking provides peace of mind that your processes are fully compliant with AS 5369 and NSQHS – Standard 3.

product families
Automated assignment of Product Families to RMD's
- Product Families are built into the tracking system providing ease of implementation.
- Product Family numbers and Steam Penetration Resistance values are printed on the barcode label.
- Process checks are performed to ensure that instruments and Trays are not processed through incompatible processes.

support when you need it
- 24-hour helpdesk
- Remote assistance support
- Education / Training support
- Back to base repairs for barcode scanners and printers
- Comprehensive training materials and videos
- Dedicated YouTube Channel – with over 100 video How To’s
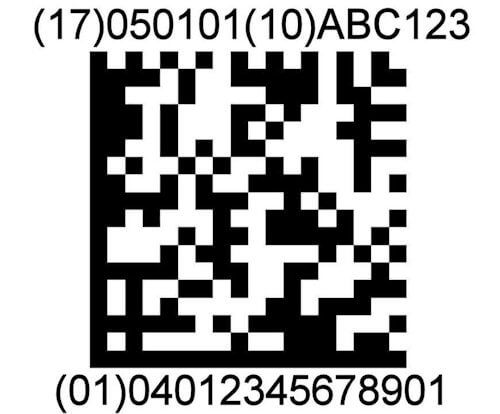
2d automation
ScanCARE Instrument Tracking supports the use of 2D data matrix barcodes on instruments. Instrument Sets can be tracked and assembled by scanning barcodes on instruments.
UDI READY
ScanCARE Instrument Tracking is UDI – GS1 Ready. The European Union and the U.S. have adopted new regulations requiring Surgical Devices to have a 2D Matrix barcode etched on them.
Australian Regulators have recommended that the EU and U.S. regulations be adopted in Australia.
prosthesis & consumable management
These days, joint replacement makes up a large part of a facilities procedure mix
The ScanCARE Activity Tracking solution provides:
- Full cost management of Prosthesis & Consumables
- Prosthesis usage tracking - know what you have, what was used and what was returned.
- Tracking and costing to Patients at the point of use
- Doctors & Anesthetist's Preference Cards
- Track usage by Specialist and Supplier
- No nasty surprise bills
- 100% live data sample size - instant reporting

