Scancare activity surgical instrument tracking system
ScanCARE is an Electronic Instrument Tracking and Traceability system that tracks and stores information about each surgical instrument as it is re-processed to its end use on a patient.
Using barcodes and scanners, it efficiently captures the entire process in real-time with ease.
ScanCARE Activity Tracking Features:
- Product Families
- Staff Competencies
- Staff Activity
- Environmental Testing
- Decontamination
- Release Criteria
- Sterilizer and Washer Validations
- Loan Sets
- Non-Conformance management and reporting
- Proof of Process
- Compatible and Preferred Cycle Types
- Record consumables and Lot numbers
- Fast Track Instruments
- ACORN compliant Count Sheets
- Paperless Count Sheets
- 2D automation and Instrument assembly
- Instrument Service and History
- HIPOT Testing
- Inter site instrument lending and tracking
- Alert system
- Audit log
- Web based application
- Attach IFU’s, Safety Data Sheets, Instrument and Tray images, Videos
the tracking system process

navigate product families with ease
Product Families are built into the system providing ease of implementation.
Product Family numbers and Steam Penetration Resistance values are printed on the barcode label.
Process checks are performed to ensure that instruments and Trays are not processed through incompatible processes.

COMPLIANCE GuaRANTEE
Designed and developed in Australia to comply with AS/NZS Standards, ScanCARE Activity Tracking provides peace of mind that your processes are fully compliant with AS 5369 and NSQHS – Standard 3.
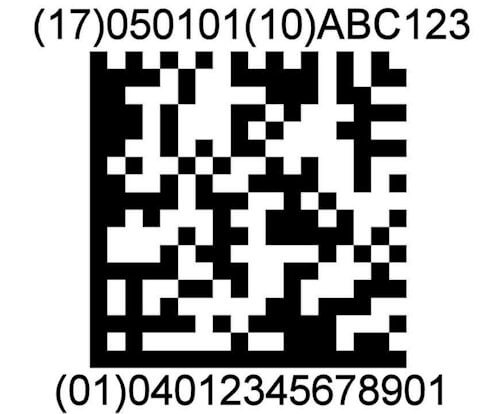
2d automation
ScanCARE Instrument Tracking supports the use of 2D data matrix barcodes on instruments. Instrument Sets can be tracked and assembled by scanning barcodes on instruments.
UDI READY
ScanCARE Instrument Tracking is UDI – GS1 Ready. The European Union and the U.S. have adopted new regulations requiring Surgical Devices to have a 2D Matrix barcode etched on them.
Australian Regulators have recommended that the EU and U.S. regulations be adopted in Australia.
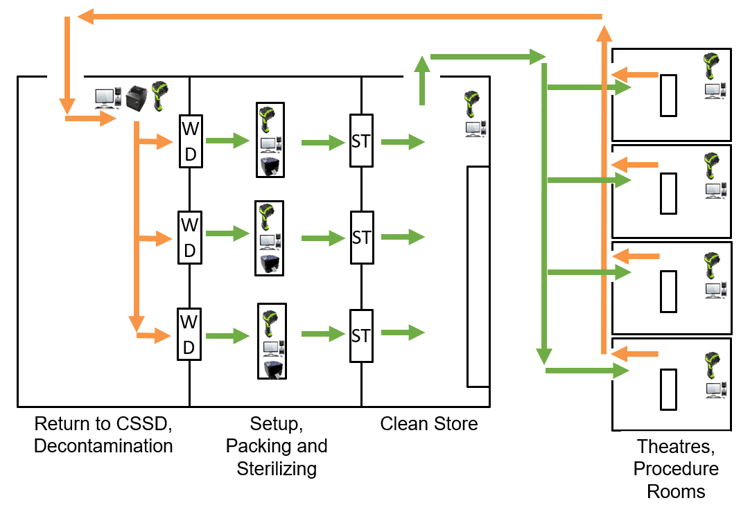
the instrument tracking pathway

Want to know how much it would cost to implement the ScanCARE Instrument Tracking System in your Facility?
Click the link below to fill out the form and we will send you an estimation.

